- This topic is empty.
-
AuthorPosts
-
10/07/2025 at 18:09 #4231
2-amino-5-bromo-4-methylpyridine (CAS No.: 98198-48-2) is an important intermediate in the field of organic synthesis and pharmaceutical development. Structurally characterized by a substituted pyridine ring, it contains three functional groups: an amino group (-NH₂) at the 2-position, a bromine atom at the 5-position, and a methyl group at the 4-position. These substituents endow the molecule with unique electronic and steric properties that make it a versatile building block for synthesizing a broad range of complex organic compounds, including biologically active molecules, agrochemicals, and heterocyclic drugs. In this blog post, SACH, a high purity industrial fine chemicals manufacturer, will share the role of organic synthesis intermediate 2-amino-5-bromo-4-methylpyridine for sale.
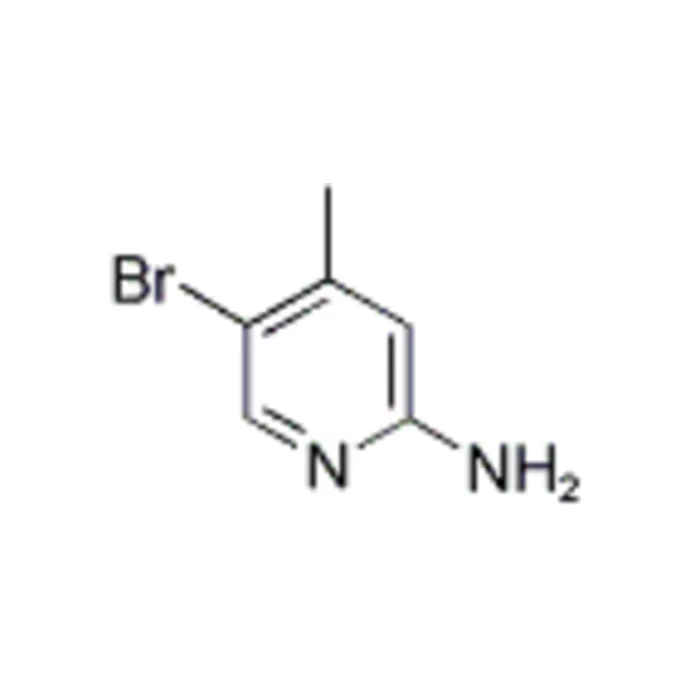
2-Amino-5-Bromo-4-Methylpyridine Structural Features and Reactivity
2-Amino-5-bromo-4-methylpyridine possesses a pyridine ring – a six-membered aromatic heterocycle with one nitrogen atom – which is a common scaffold in medicinal chemistry. The amino group at the ortho position relative to the nitrogen atom provides nucleophilic character, often enabling coupling reactions such as Buchwald-Hartwig aminations and amidations. The bromine atom at the 5-position introduces electrophilic reactivity, rendering the molecule suitable for palladium-catalyzed cross-coupling reactions like Suzuki-Miyaura, Stille, or Heck couplings. The methyl group at the 4-position subtly modulates the electron density of the ring, thereby influencing reactivity and regioselectivity in further synthetic transformations.
Applications of 2-Amino-5-Bromo-4-Methylpyridine in Medicinal Chemistry
One of the most significant applications of 2-amino-5-bromo-4-methylpyridine lies in the design and development of pharmaceuticals. This compound is often used as a core intermediate in the synthesis of drug candidates due to its heteroaromatic scaffold and multiple reactive handles.
1. Heterocyclic Drug Development
The pyridine ring is a key pharmacophore in many therapeutic agents because of its ability to mimic natural heterocycles and form hydrogen bonds with biological targets. The brominated amino methylpyridine serves as a precursor to fused heterocyclic systems such as imidazopyridines, pyridopyrimidines, and triazolopyridines, which are known for a wide range of bioactivities including:
* Anticancer
* Antiviral
* Anti-inflammatory
* Antimicrobial properties
For instance, by modifying the amino group to form amide or urea derivatives and engaging the bromo position in Suzuki coupling to introduce aryl groups, medicinal chemists can rapidly generate analog libraries for structure-activity relationship (SAR) studies.
2. Kinase Inhibitor Scaffold
2-Amino-5-bromo-4-methylpyridine can be utilized in the synthesis of kinase inhibitors, particularly targeting protein kinases implicated in cancer and autoimmune disorders. The amino group interacts with the ATP-binding site of kinases, and selective substitution at the 5-position enables binding pocket specificity.
3. CNS-Active Compounds
Pyridine derivatives also show promise in crossing the blood-brain barrier, making them suitable for central nervous system (CNS) drug development. Molecules derived from 2-amino-5-bromo-4-methylpyridine have been explored as potential neuromodulators, antidepressants, or neuroprotective agents.
Applications of 2-Amino-5-Bromo-4-Methylpyridine in Agrochemical Synthesis
In the field of agrochemicals, 2-amino-5-bromo-4-methylpyridine is used as an intermediate for synthesizing herbicides, fungicides, and insecticides. Heterocyclic scaffolds like substituted pyridines are essential for designing molecules with high selectivity and potency against plant pathogens or pests.
Functional derivatization of the amino and bromo positions allows for the generation of analogs with desired physicochemical properties such as water solubility, environmental stability, and target specificity. These properties are crucial for ensuring efficacy while minimizing environmental impact and resistance development in target organisms.
Role of 2-Amino-5-Bromo-4-Methylpyridine in Organic Synthesis
In synthetic organic chemistry, this compound serves as a multipurpose reagent for elaborating more complex molecules due to the following characteristics:
1. Versatility in Cross-Coupling Chemistry
The presence of the bromine atom on the pyridine ring allows for palladium-catalyzed cross-coupling reactions. This enables the introduction of various substituents such as:
* Aryl groups (via Suzuki coupling)
* Alkynyl or alkenyl groups (via Sonogashira or Heck reactions)
* Alkyl groups (via Negishi or Kumada couplings)
Such transformations are critical in combinatorial chemistry and high-throughput screening where rapid synthesis of molecular libraries is required.
2. Nucleophilic Substitution and Functionalization
The amino group at position 2 can undergo:
* Acylation to form amides
* Sulfonylation to yield sulfonamides
* Reductive alkylation or arylation
These transformations expand the diversity of accessible functional molecules and are often applied in lead optimization during drug discovery.
3. Scaffold for Fused-Ring Systems
By using appropriate ring-closing reactions (such as cyclocondensation or intramolecular nucleophilic substitution), this intermediate enables the formation of polycyclic aromatic compounds, which are of particular interest in both pharmaceuticals and material science.
2-Amino-5-Bromo-4-Methylpyridine Process Development and Scalability
For industrial applications, particularly in pharmaceutical manufacturing, the scalability and availability of intermediates like 2-amino-5-bromo-4-methylpyridine are critical.
1. Synthetic Accessibility
This compound is commercially available or can be synthesized in multi-gram to kilogram scale using methods such as:
* Electrophilic bromination of 4-methylpyridine derivatives
* Nitration followed by reduction to introduce the amino group
High-yielding and reproducible synthetic protocols are essential for minimizing production cost and ensuring quality control.
2. Purity and Stability
The chemical stability of 2-amino-5-bromo-4-methylpyridine under standard storage conditions makes it suitable for long-term use in research and industrial settings. High purity grades (>98%) are typically required for pharmaceutical-grade synthesis to avoid downstream purification complexities.
Analytical Characterization
Accurate characterization of this intermediate is essential for its use in regulated industries like pharmaceuticals.
* NMR Spectroscopy (¹H and ¹³C NMR): Confirms the presence and position of methyl, amino, and bromo substituents.
* Mass Spectrometry (MS): Provides molecular weight verification.
* HPLC and GC: Assess purity and monitor synthetic transformations.
* IR Spectroscopy: Detects functional groups such as NH₂ and C-Br stretches.
These analytical tools ensure structural integrity and consistency across production batches.
Environmental and Safety Considerations
Though widely used, the handling of 2-amino-5-bromo-4-methylpyridine requires compliance with safety standards:
* It should be manipulated in a well-ventilated fume hood due to potential irritation from volatile amines.
* Appropriate PPE (personal protective equipment) including gloves, goggles, and lab coats should be worn.
* Disposal should adhere to local environmental regulations due to the presence of halogenated aromatic systems that may pose ecological risks.
Conclusion
2-Amino-5-bromo-4-methylpyridine (CAS No.: 98198-48-2) serves a pivotal role in organic synthesis, particularly in the development of pharmaceuticals and agrochemicals. Its unique combination of reactive functional groups and a modifiable heteroaromatic scaffold allows chemists to access a diverse range of bioactive and functionalized molecules.
-
AuthorPosts
- You must be logged in to reply to this topic.